Abstract
AlloSCT from HLA-matched MSD has been successful for high-risk SCD, and is the only known curative therapy (Talano/Cairo et al EJH, 2014). We have recently demonstrated 100% EFS and absence of sickle cell symptoms following reduced toxicity conditioning in HLA matched sibling donor (MSD) or cord blood AlloSCT (Bhatia/Cairo et al BMT, 2014). However, 5 out of 6 children lack an HLA MSD without SCD. Only 19% of recipients of African descent will identify a well-matched unrelated donor (MUD) and results after unrelated UCBT are poor (Radhakrishnan/Cairo et al BBMT, 2013). Recent results utilizing matched unrelated donors in a multi-center trial showed unacceptable rates of cGVHD at 62% (95% CI 41-77) (Shenoy et al Blood, 2016). We demonstrated CD34+ selection followed by T-cell addback from MUD in pediatric recipients led to 100% engraftment with minimal aGVHD (Geyer/Cairo et al BJH, 2011). Limitations in the past of various T-cell depletion methods have included a higher incidence of graft failure, delayed immune reconstitution, opportunistic fungal infection and higher incidence of cGVHD. FHI TCD AlloSCT utilizing the approach of CD34+ enrichment and T-cell addback could expand the donor pool and improve outcomes for patients with high risk SCD and have similar outcomes to AlloSCT from HLA MSD.
This SCD consortium trial (www.sicklecelltransplantconsortium.org) (NCT01461837) is investigating the safety, feasibility, EFS, donor chimerism, graft failure, aGVHD and cGVHD after FHI TCD AlloSCT in high-risk SCD patients.
High risk included: ≥1 CVA, ≥2 ACS, ≥3 VOC in past 2 years, or 2 abnormal TCDs. Patients (2-<21 yrs) who have ≥1 high-risk SCD features were eligible. Patients received hydroxyurea and azathioprine, day -59 - day -11, fludarabine (150mg/m2), busulfan (12.8mg/kg) (targeted trough Css of 600-900), thiotepa (10 mg/kg), cyclophosphamide (200mg/kg), R-ATG (8mg/kg), and TLI (500cGy) followed by FHI T-cell depleted AlloSCT. AGVHD prophylaxis: tacrolimus. We utilized the CliniMACS to enrich for peripheral blood HPC's; target dose of 10 x 106 CD34+ cells/kg with a fixed dose of 2 x 105 CD3+ T cells/kg. The Child Health Rating Inventories (CHRIs) was used to serially collect parent proxy-rated health-related quality of life (HRQL).
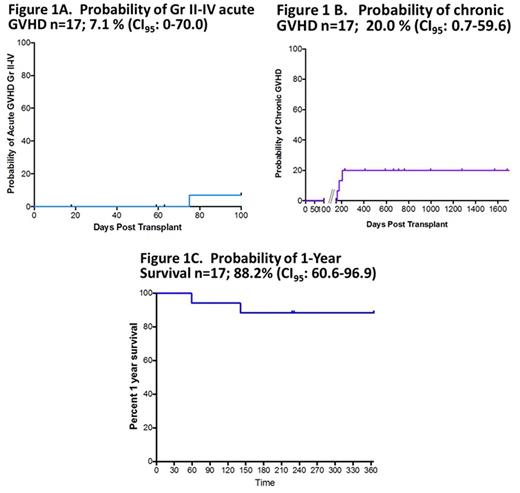
Twenty-one patients have been enrolled. Nineteen patients have received AlloSCT to date. Fifteen patients utilized maternal donors and 4 patients utilized paternal donors. Two donors encountered thrombocytopenia that resolved spontaneously. The mean ± SD CD34+ enrichment prior to cryopreservation was 12.66 ± 3.3 x 106/kg and log T-cell depletion was 4.8 ± 0.58. Evaluable pts had early neutrophil engraftment (median day +9), and ≥95% whole blood and RBC (CD71) donor chimerism at 1yr. Probability of aGVHD n=17, 7.1% (CI95: 0-70.0); cGVHD n=17, 20% (CI95: 0.7-59.6) (Figure 1A and 1B). Probability of 1-Year Overall Survival (OS) n=17; 88.2% (CI95: 60.6-96.9) (Figure 1C). Immune reconstitution was robust with 1 yr NK, CD3, CD4 and CD8 204±37 cells/μL, 795±168 cells/μL, 408±102 cells/μL and 375±90 cells/μL, respectively. Two-yr PFT results were also robust with % predicted FVC, FEV1, TLC and DLCO of 89%, 84%, 82%, and 68%, respectively. All survivors are free of SCD symptoms and without signs of progression of multi-organ toxicity. One patient developed late hepatic SOS and died at day +59; one patient died at day +390 due to complications of CGVHD, and one patient died at day +141 of steroid refractory aGVHD, the remainder are alive and free of SCD symptoms with a median follow-up of +711 days (range, +231 to +1684 days). Most importantly, mean + SD HRQL score (0-100) reported at 1 yr for emotional, physical functioning, role, and global scores were 82.9±9.7 points, 82.8±15.8 points, 89.6±13.3 points, and 86±8.2 points, respectively.
Early results indicate promising results in 1 yr OS, stable donor chimerism, and low incidence of acute and/or cGVHD after FHI HCT utilizing CD34 enrichment and CD3/MNC addback in high-risk SCD patients who lack a MSD or URD. A longer term follow-up is needed to assess long-term safety and outcomes. This research was supported by RO1FD004090-01A1.
Walters: AllCells, Inc: Other: Medical Director; bluebird bio: Research Funding; Sangamo Therapeutics: Consultancy; ViaCord Processing Lab: Other: Medical Director. Cairo: Jazz Pharmaceuticals: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal